The maximum number of electrons that an energy level can hold is determined from the formula 2n^2 equals the total number, where n is the energy level. Thus, the first energy level holds 2. 1^2 = 2 electrons, while the second holds 2. 2^2 = 8 electrons. With the help of the periodic table, we can easily see that the atomic number of helium is 2. As its atomic number is 2, it has two protons, and for neutral helium, the number of protons is always equal to the number of electrons i.e. Have two-electron in their nucleus. Step 2: Write Electron Configuration.
How are the elements differentiated? And how many protons occur in hydrogen, helium, and lithium?
3 Answers
Explanation:
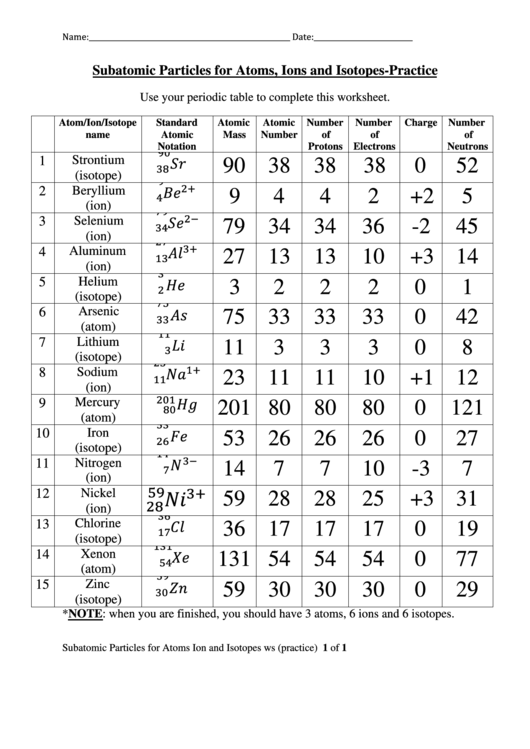
The number of protons is the same as the atomic number (hydrogen is one, helium two, lithium three etc.) and the number of electrons is the same as the number of protons (assuming the atom is neutral.)
The more tricky bit is figuring out the number of neutrons. You do this by finding the mass number from the periodic table and subtracting the atomic number (the number of protons you found earlier.) Thus hydrogen has zero neutrons, helium has 2 (the mass 4, minus the no. of protons, 2), lithium has 4 (7 - 3) etc.
A quick check is to find the number of protons uranium-238 has. If you get the number 146, you've sussed it.
The periodic table gives an atomic number and weight for each element.
The atomic number is the number of protons in an atom of the element.
The atomic weight equals (very nearly) the number of protons plus the number of neutrons. Subtract the atomic number from the atomic weight and you'll be very close to the number of neutrons.
(but see the exception noted below)
The number of electrons will usually equal the number of protons. This is in a 'normal', electrically neutral charged atom. Atoms often give up one or more electrons (or accept an additional one or more electrons), in which case they are 'ions'.
Atoms may also have more (or fewer) neutrons, in which case they are called 'isotopes' of the element. 'Deuterium', for example, is an isotope if hydrogen with an extra neutron. Chemically, these atoms behave the same as for the normal version of the element. Atomic number is the same, but atomic weight will be greater (or less) than the atomic weight given on the periodic table.
Whew. Haven't read up on this stuff for years. You now know everything I know on this subject.
An element on the Periodic Table is characterized by its chemical symbol, which is specific to its

Explanation:
And have you got a Table handy? Of course you have because you are doing your chemistry homework.
Now
The nucleus also contains massive, neutrally charged particles, i.e.
The nucleus of given element can contain various number of neutrons, and this gives rise to the phenomenon of isotopes. These are nuclei of the same element (because
I will give you one example of common isotopes. Hydrogen comprises (as far as we know) maybe 70% of the matter in the universe. Most hydrogen atoms contain only the one proton, i.e. we gots
Confused yet? Remember chemists and physicists are simple folk, and I don't think these concepts are entirely off the wall and inaccessible.
And see this old answer.
Related questions
Click to see full answer
Also question is, how many energy levels do the elements in the 2nd row fill up?
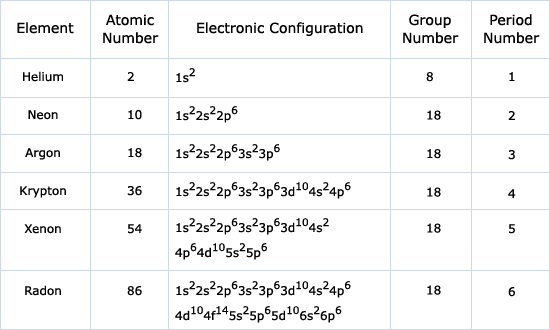
When you look at the Periodic Table of the Elements, the energy levels of the atoms correspond to the rows of the table. The two elements in the top row, hydrogen and helium, are filling their first energy level with their final electrons. The eight elements of the second row are filling their second energy level.
Number Of Protons For Helium
Secondly, how many energy levels are in hydrogen? The formula defining the energy levels of a Hydrogen atom are given by the equation: E = -E0/n2, where E0 = 13.6 eV (1 eV = 1.602×10-19 Joules) and n = 1,2,3… and so on.
Similarly, you may ask, how many energy levels are in helium?
| Element | Element Number | Number of Electrons in each Level |
|---|---|---|
| Helium | 2 | 2 |
| Lithium | 3 | 2 |
| Beryllium | 4 | 2 |
| Boron | 5 | 2 |
Which element has the highest energy level?
The two electrons of helium, for example, are confined to a spherical zone surrounding the nucleus called the K shell or K energy level. Lithium (At. No. = 3) has three electrons, two in the K shell and one located farther from the nucleus in the L shell.
helium, for example, are confined to a spherical zone surrounding the nucleus called the K shell or K energy level. Lithium (At. No. = 3) has three electrons, two in the K shell and one located farther from the nucleus in the L shell.Helium Element Number Of Electrons
Electrons.Atomic Mass Of Helium
| Atomic Number | |
|---|---|
| Element | |
| Energy Levels or 'shells' | K |
| L | |
| M | |
